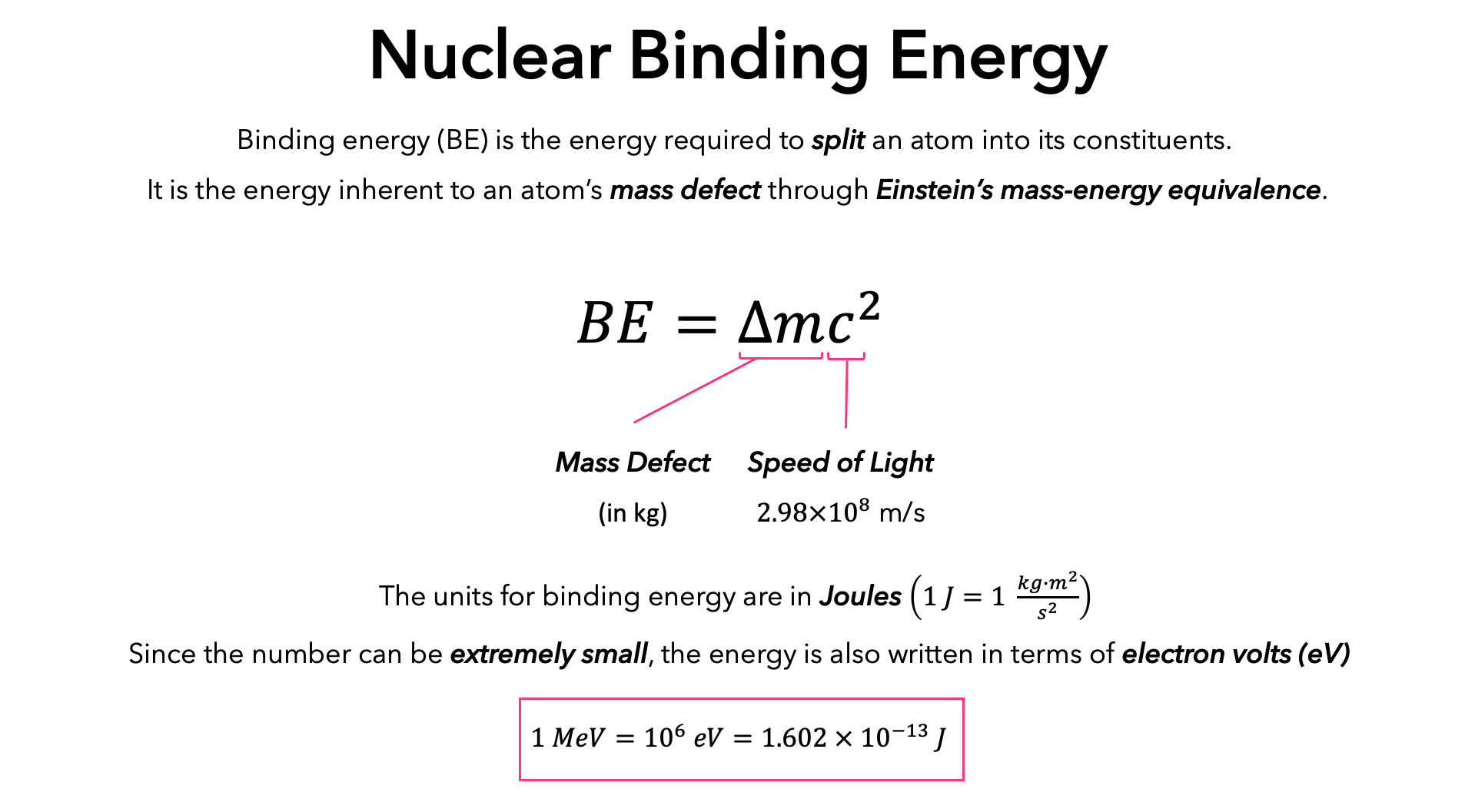
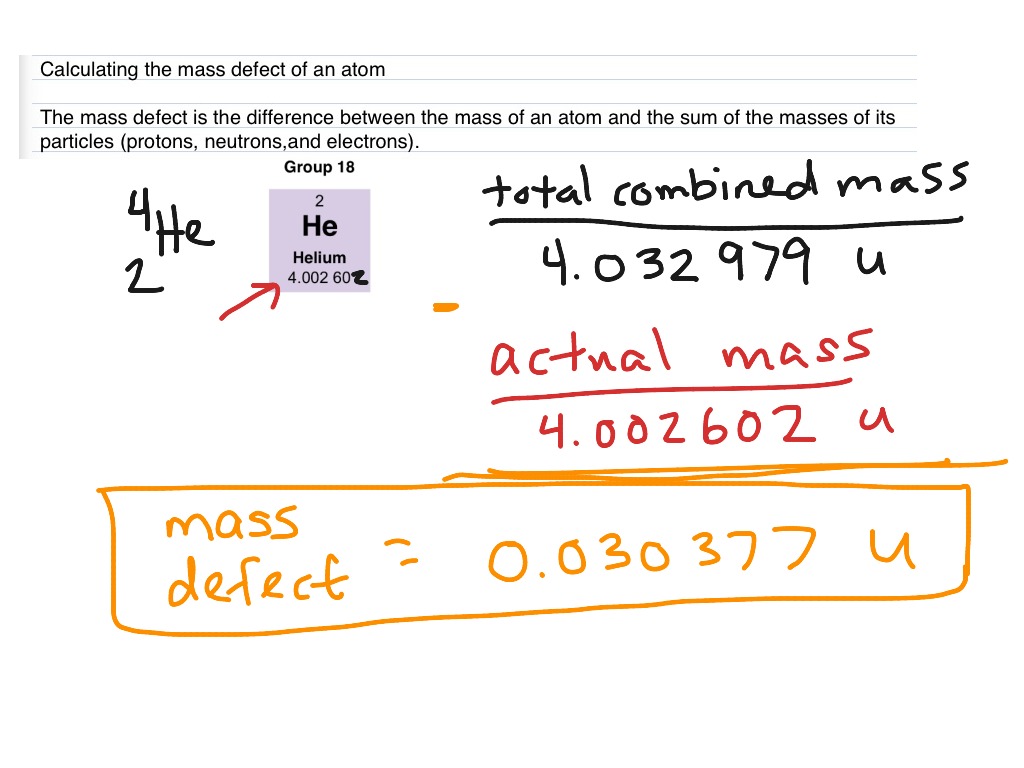
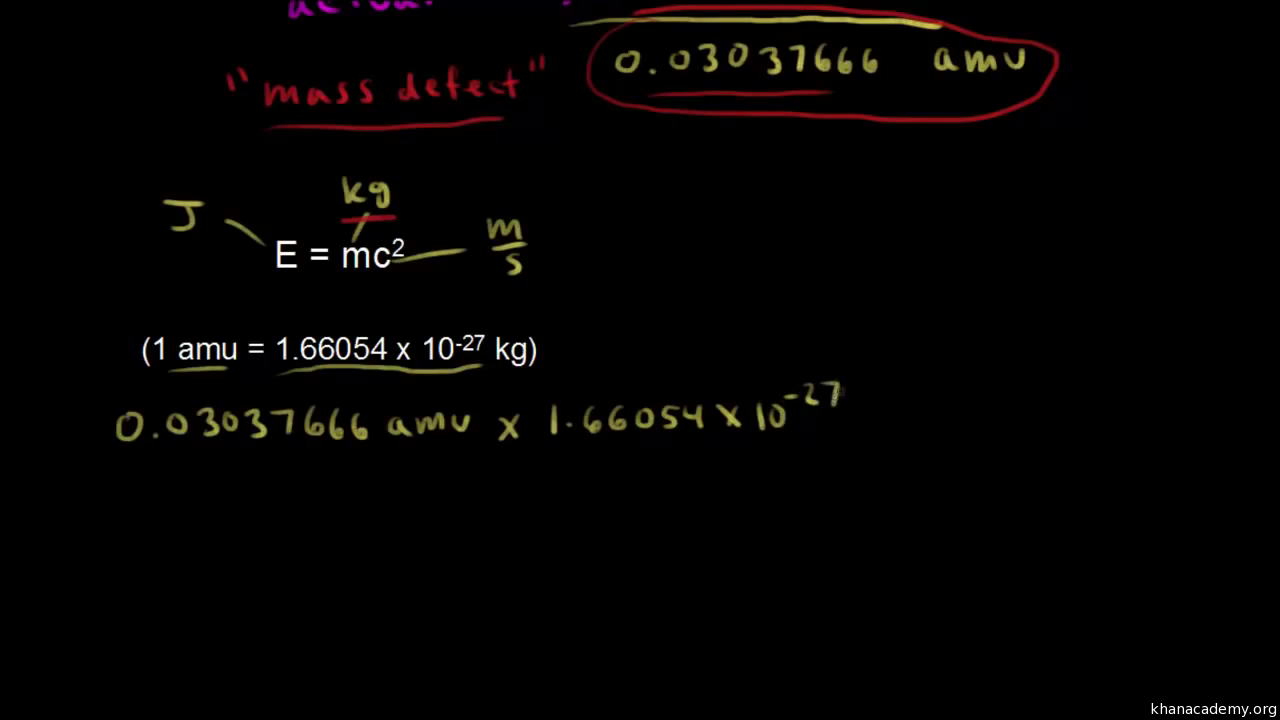![Calculate the mass defect and the binding energy per nucleon of the ""(47)^(108)Ag nucleus. [atomic mass of Ag = 107.905949] Calculate the mass defect and the binding energy per nucleon of the ""(47)^(108)Ag nucleus. [atomic mass of Ag = 107.905949]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/378267553_web.png)
Calculate the mass defect and the binding energy per nucleon of the ""(47)^(108)Ag nucleus. [atomic mass of Ag = 107.905949]

A web application to calculate the mass defect and nuclear binding energy per nucleon - ScienceDirect
Calculate mass defect and binding energy per nucleon of 2010Ne, given. - Sarthaks eConnect | Largest Online Education Community
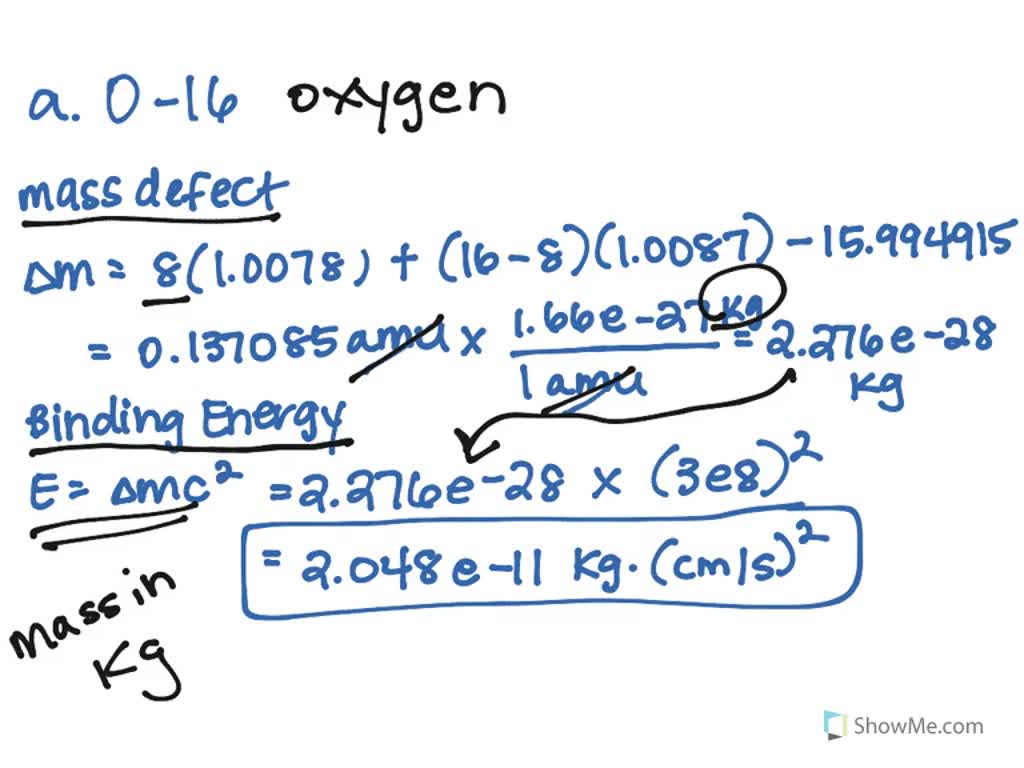
SOLVED:Calculate the mass defect and nuclear binding energy per nucleon of each nuclide. a. O-16 (atomic mass =15.994915 amu) b. Ni-58 (atomic mass =57.935346 amu) c. Xe-129 (atomic mass =128.904780 amu)

Calculate the (i) mass defect, (ii) binding energy and (iii) the binding energy per nucleon for a 6C^12 nucleus. Nuclear mass of 6C^12 = 12.000000 a.m.u., mass of hydrogen nucleus = 1.007825

How do you calculate the mass defect and nuclear binding energy per nucleon of each of the nuclides? | Socratic
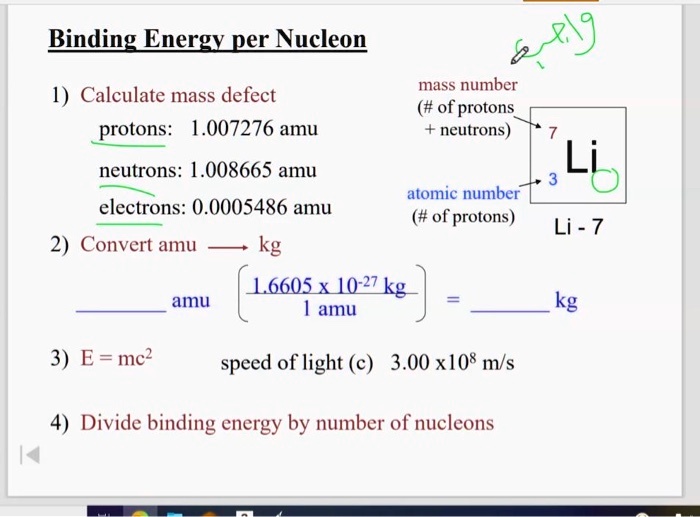
SOLVED: Binding Energy perNucleod mass number (# of protons neutrons) 1) Calculate mass defect protons: 1.007276 amu neutrons: 1.008665 amu atomic number electrons: 0.0005486 amu (# of protons) 2) Convert amu kg