
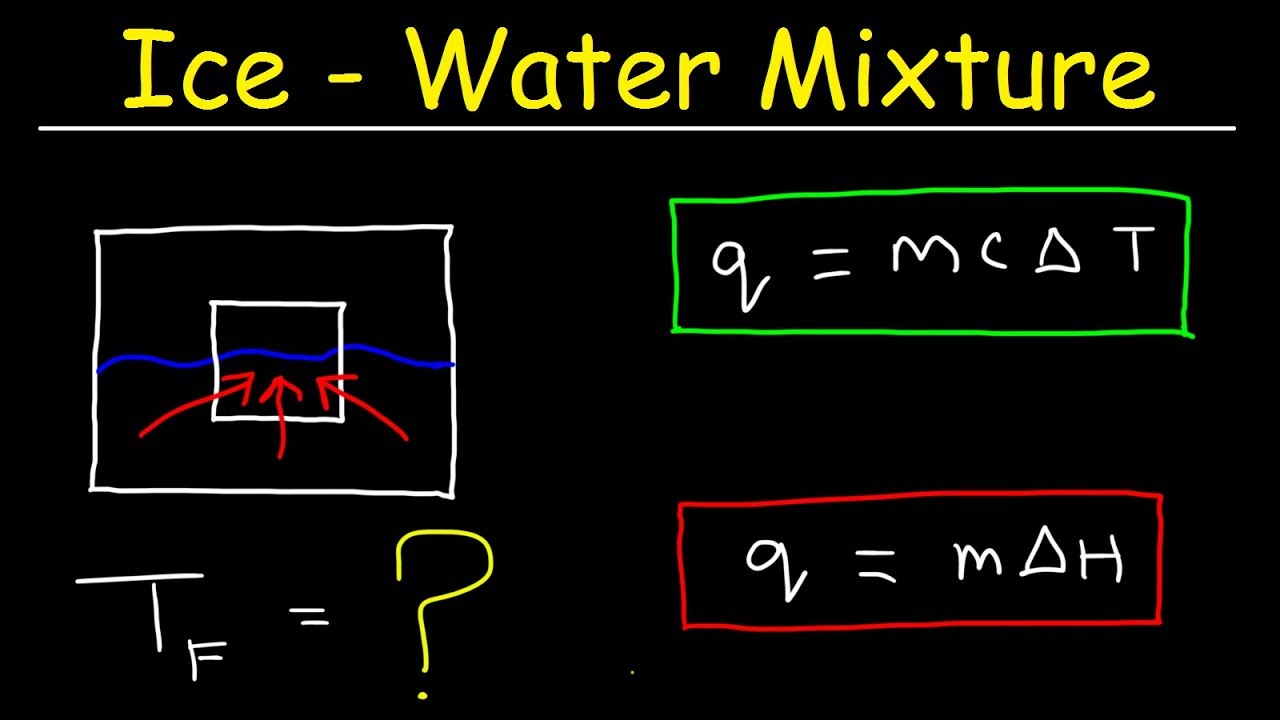
Calculate the enthalpy change on freezing of `1 mol` of water at `10^(@)C` to ice at `-10^(@)C`. - YouTube

SOLVED:Use standard enthalpies of formation to calculate the standard change in enthalpy for the melting of ice. (The ΔHf^∘ for H2 O(s) is -291.8 k k / / mol . ) Use

Calculate the heat of fusion of ice from the following data of ice at `0^@C` added to water. Mass of - YouTube

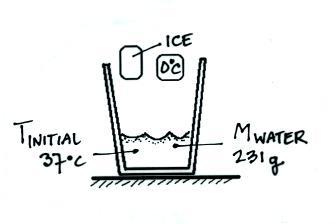
4 kg of ice at-20^(@)C is mixed with 10 kg of water at 20^(@)C in an insulating vessel having a negligible heat capacity. Calculate the final mass of water remaining in the
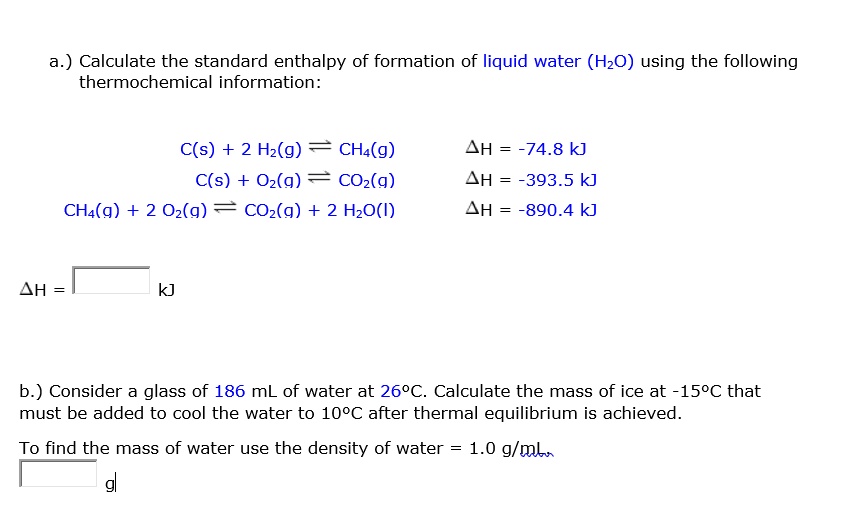
SOLVED: a.) Calculate the standard enthalpy of formation of liquid water (HzO) using the following thermochemical information: C(s) + 2 Hz(g) CHa(g) C(s) Oz(g) COz(g) CHa(g) + 2 Oz(g) COz(g) + 2



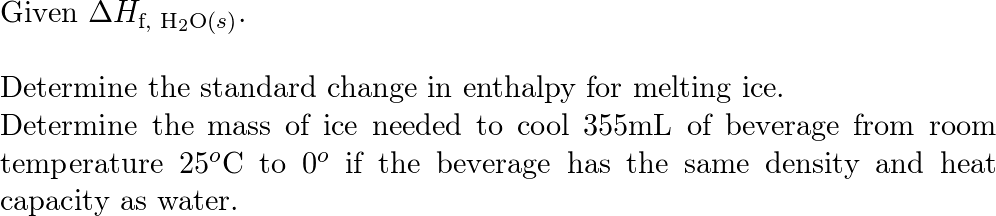

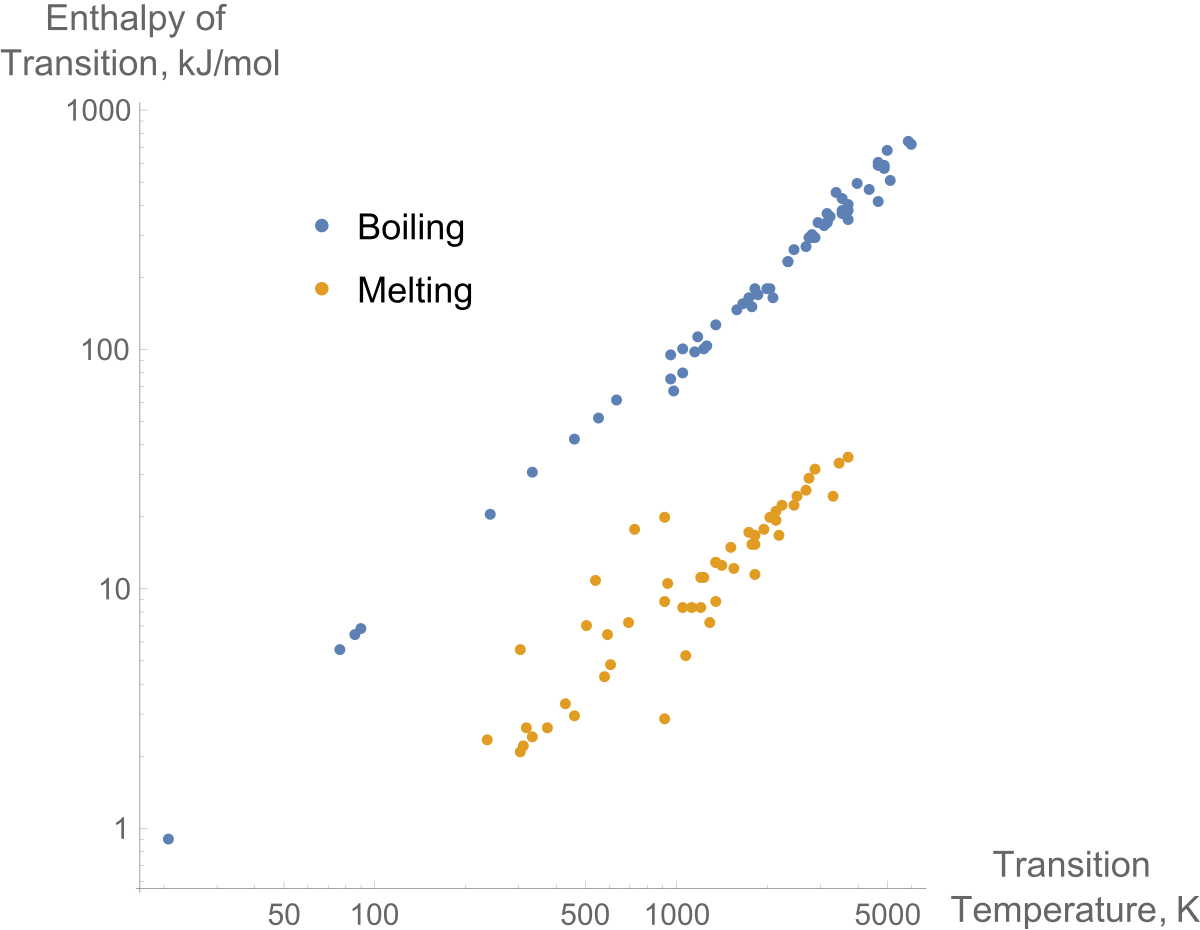
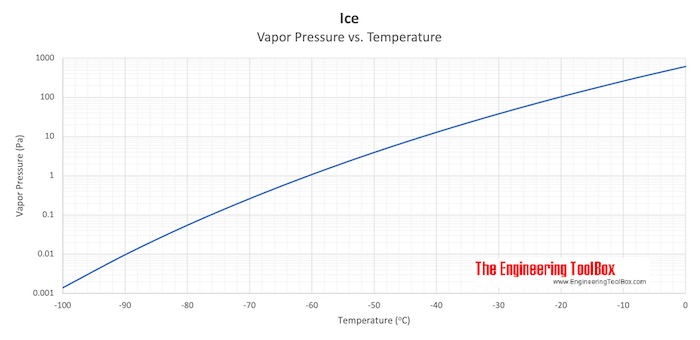
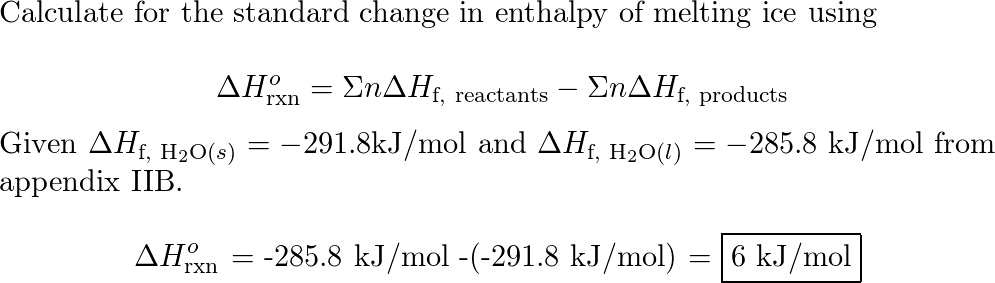

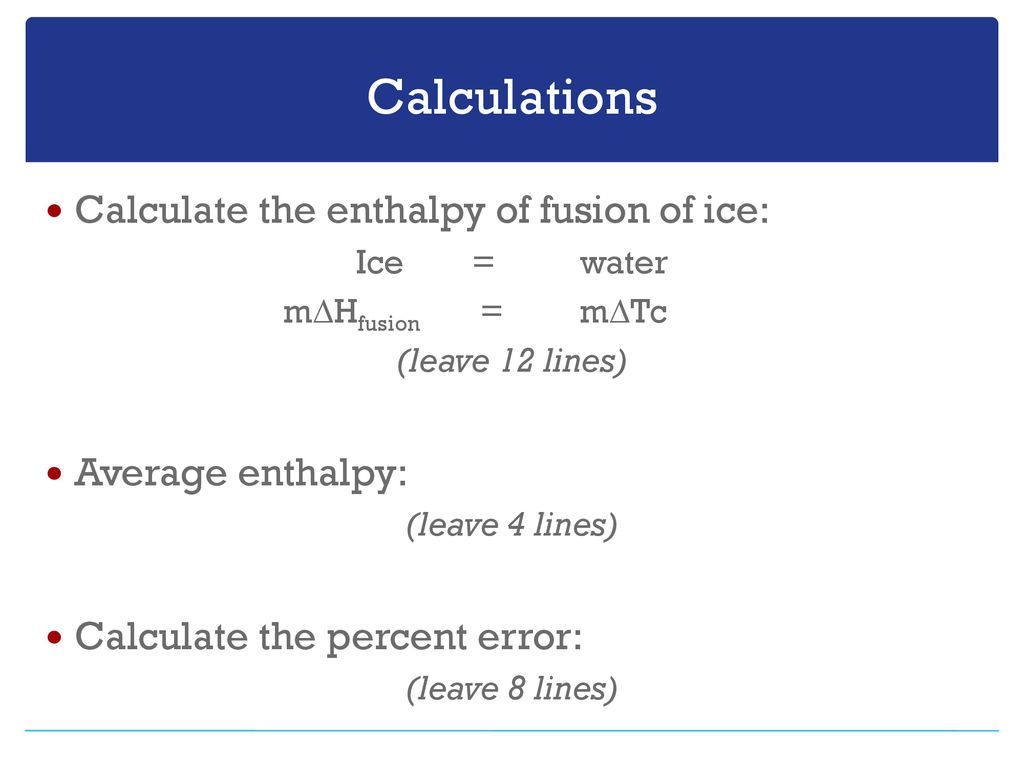



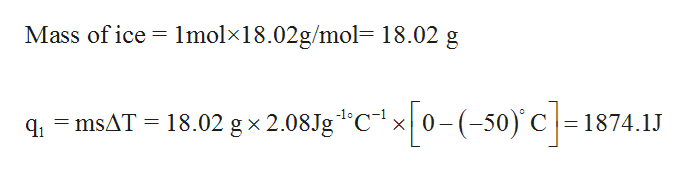
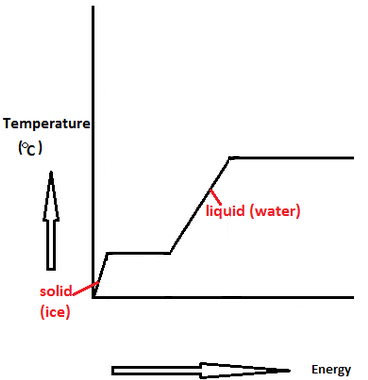
:max_bytes(150000):strip_icc()/GettyImages-1070123348-c9a136e91e7f4b6f9848581aa28b26d1.jpg)


