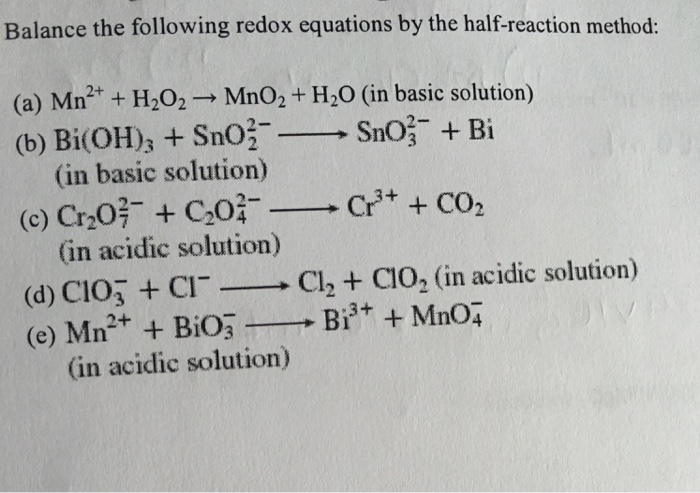
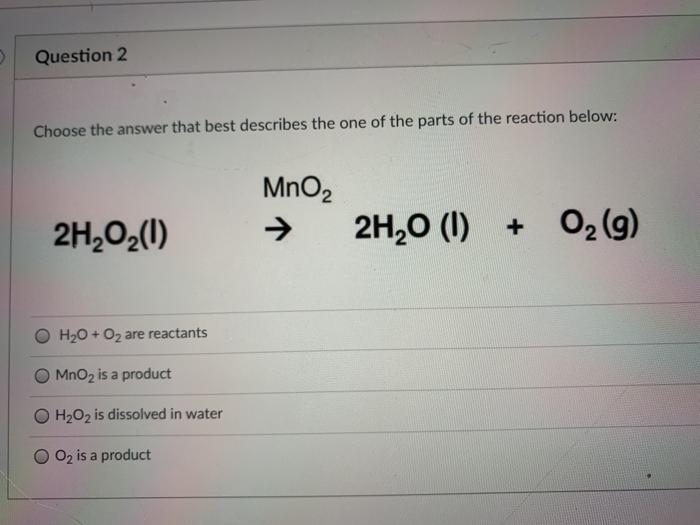
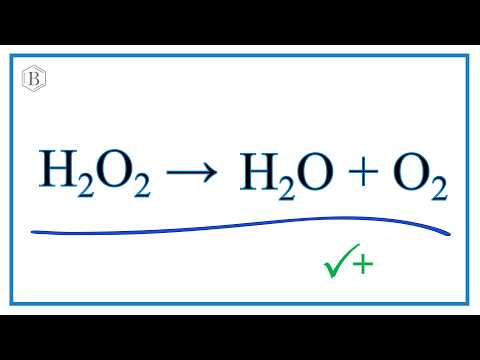
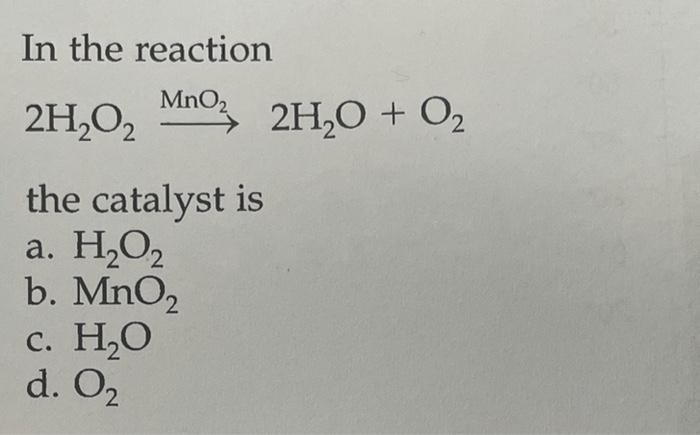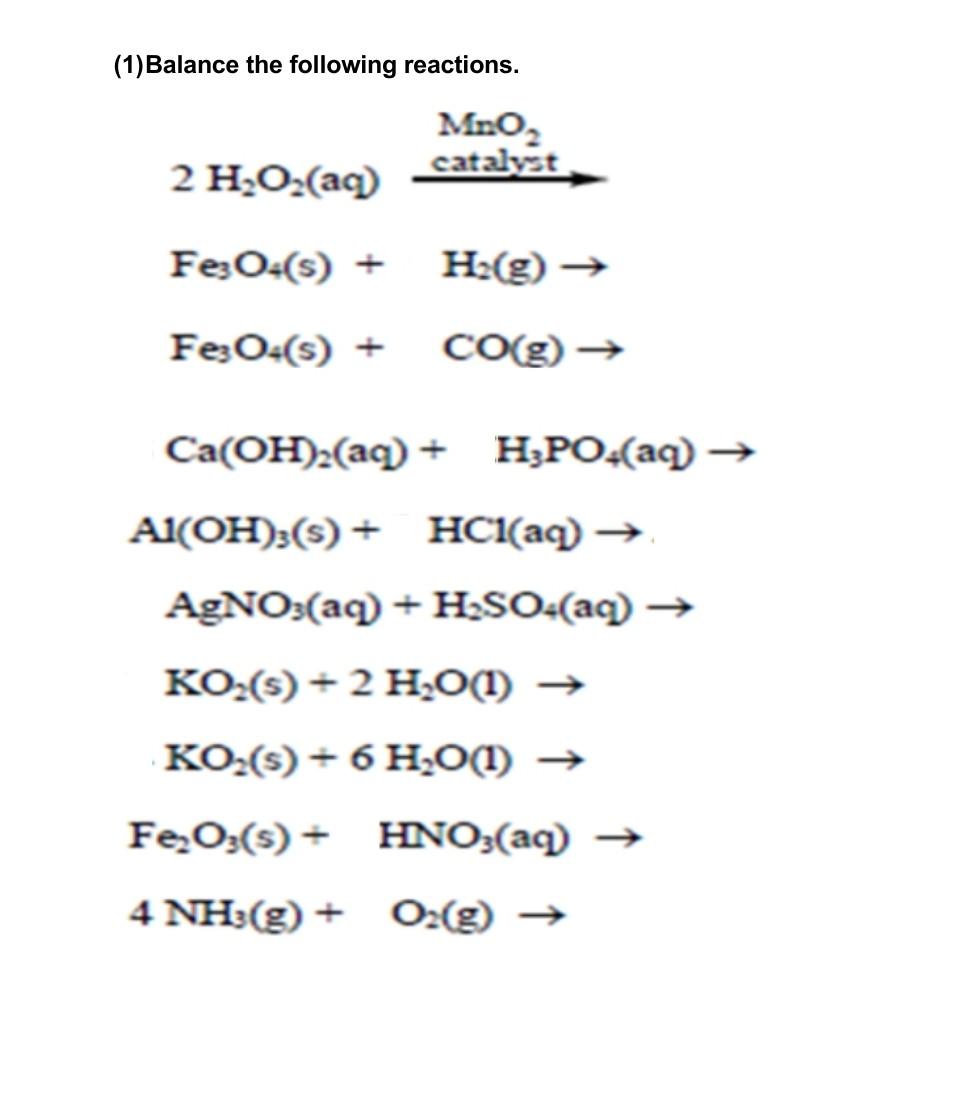SOLVED: Mn2+ + H2O2 → MnO2 + H2O in basic medium Separate the reaction into two half-reactions and balance each of them.

Fluorometric methods for determination of H2O2, glucose and cholesterol by using MnO2 nanosheets modified with 5-carboxyfluorescein | SpringerLink
Study of Hydrogen Peroxide Reactions on Manganese Oxides as a Tool To Decode the Oxygen Reduction Reaction Mechanism

inorganic chemistry - Reaction intermediates of MnO2 catalyzed H2O2 decomposition reaction - Chemistry Stack Exchange

Does the reaction rate depend on the concentration of the catalyst? For example, I found that the decomposition of H2O2 stops if we add excess MnO2. Are there any other examples like

K956: Catalysis – MnO2 catalyzed decomposition of H2O2 (“Genie in a Bottle”) | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

IA on effectiveness of different types of catalysts MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor.

Formation and Oxidation Reactivity of MnO2+(HCO3–)n in the MnII(HCO3–)–H2O2 System | Inorganic Chemistry

inorganic chemistry - Reaction intermediates of MnO2 catalyzed H2O2 decomposition reaction - Chemistry Stack Exchange

Facile preparation of MnO2–TiO2 nanotube arrays composite electrode for electrochemical detection of hydrogen peroxide - ScienceDirect